usp class vi vs iso 10993
Typically the terms USP Class VI or ISO 10993 materials are used. However Class VI also requires subacute toxicity and implantation.
Nylon Membrane Oem Membranes And Devices Cobetterfiltration
So does ISO 10993.
. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. The most stringent Class VI requires three types of tests. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI.
A more rigorous standard for the biological. Rob Pruyn August 5 2020 Custom Products. ISO 10993 USP Class VI demands an intracutaneous irritation test.
USP Class VI demands an intracutaneous irritation test. However Class VI also requires subacute toxicity and implantation. USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the.
Rob Pruyn August 5 2020 Custom. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10. Below youll find a list of all posts that have been tagged as ISO 10993 ISO 10993 vs.
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for. Medical Molding and Biocompatible Rubber. Inside Rubber Magazine Profiles The Rubber.
Biocompatibility - USP Class VI vs. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of. So does ISO 10993.
Steve Melito August 5 2020. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. A number of our plastic materials are ISO-10993 or USP Class VI capable.
USP Class VI vs. USP Class VI and ISO 10993. A number of our plastic materials are ISO-10993 or USP Class VI capable.
In an effort to standardize biocompatibility testing worldwide the International Standards Organization ISO developed ISO 10993. Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs. Take an ASTM D2000 call out.
ISO 134852016 - Medical Device Quality Management Systems. You might establish biocompatibility via making the device of a Recognized Consensus. May 1 2009.
Medical Molding and Biocompatible Rubber. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the. USP class VI versus ISO 10993.
USP Class VI vs. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10. If yes to the first question then USP Class VI is not a relevant qualification for it.
Iso 10993 vs. ISO 10993 is a 20-part standard that. Testing for proving food safety on USP class.
Unlike other rubber standards theres no one standard that engineers use for an approval.
General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Chemical Characterization And Non Targeted Analysis Of Medical Device Extracts A Review Of Current Approaches Gaps And Emerging Practices Acs Biomaterials Science Engineering
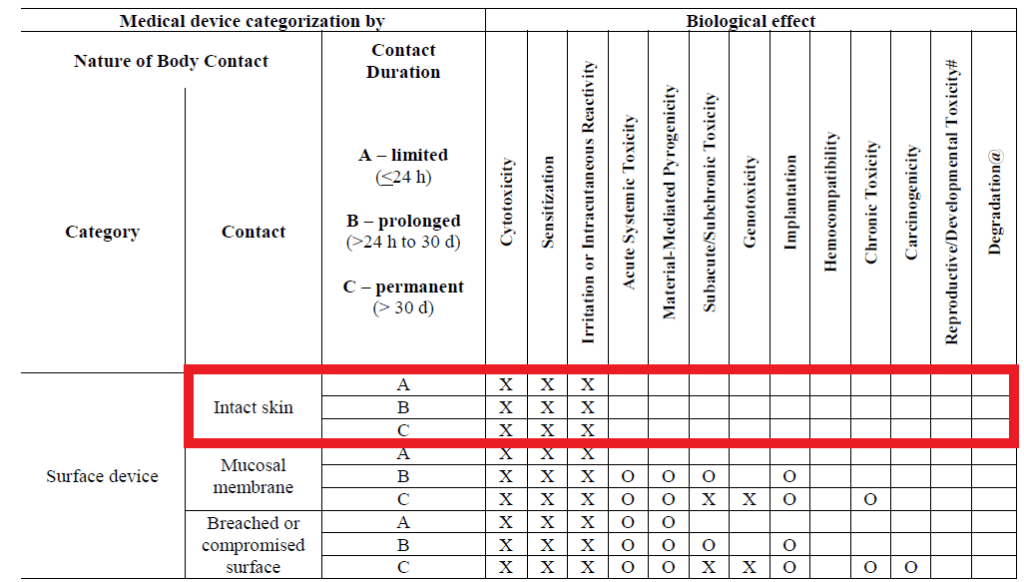
Fda Guidance Device Biocompatibility Intact Skin Namsa

Duran Pure Premium Lip Seal Screw Caps Pfa Unlined Gl45 Pack 5
Medical Device Adhesives Panacol Usa

Thermoplastic Elastomers For Medical Devices Meet Usp Class Vi And Iso 10993 Standards Medical Design And Outsourcing

What Are Lunacup Menstrual Cups Made Of

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi
Biocompatibility Planning Tool Biopt Pacific Biolabs

Heat Resistant Thermoplastic Ultem 9085 Baltic3d Eu

Pc Iso Polycarbonate Iso 10993 Usp Class Vi 3d Printing Material Xometry Europe

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi
Beginners Guide To Biocompatibility For Medical Device Adhesives Krylex
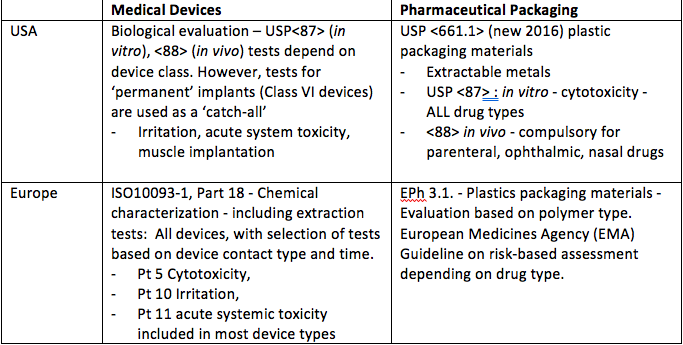
Making Plastics Safe For Medical Devices Medical Plastics News

Printing The Strongest 3d Parts With Ultem 1010 Trimech
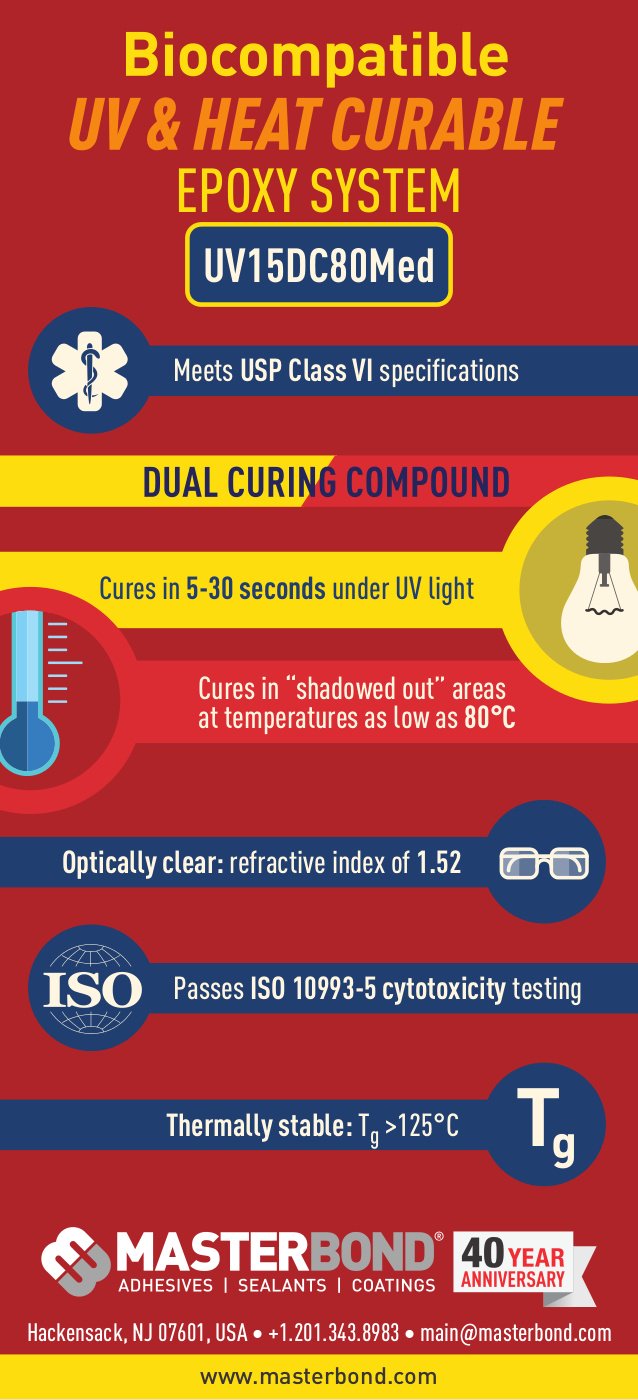
Master Bond On Twitter Infographic On Uv15dc80med A Usp Class Vi Uv And Heat Curable Epoxy Compound That Also Passes Iso 10993 5 Cytotoxicity Testing Https T Co Datfqi6lqs Https T Co Kfegr9p9w1 Twitter

